
Ever wondered why aluminum is everywhere—from soda cans and kitchen foil to airplane wings and car bodies? The answer lies in its unique set of properties, with one of the most important being its melting point. But what exactly does "melting point" mean, and why should you care about the melting point of aluminum in celsius or any other unit?
The melting point is the specific temperature at which a solid material turns into a liquid. For metals like aluminum, this temperature marks a critical threshold. Once reached, the metal changes from a rigid, structured solid to a free-flowing liquid. This transition is more than just a scientific curiosity—it’s a key factor in how materials are processed and used in real-world applications.
This guide will unlock everything you need to know about the melting point of aluminum, including:
By the end, you’ll understand not only the numbers but also the science and practical significance behind the melting point of aluminum. Whether you’re a student, engineer, or simply curious, this knowledge will help you appreciate why aluminum is such a vital material in everyday life and advanced industries alike.
When you ask, "What is the melting point of aluminum?" the answer is both precise and fundamental to countless industries. The melting point of pure, elemental aluminum is 660.32°C (or 1220.58°F)—a value established through extensive scientific measurement and recognized worldwide as a physical constant.
Imagine you’re designing a component that must withstand high heat, or you’re exploring materials for casting and fabrication. Knowing the exact temperature at which aluminum transitions from solid to liquid is crucial. This temperature is not just a guideline—it’s a benchmark that helps engineers, manufacturers, and researchers make informed decisions about processing, safety, and suitability for specific applications.
It's important to note that while the melting point of aluminum is a single, well-defined number for the pure element, alloys introduce complexity. For example, common aluminum alloys used in construction, transportation, and packaging have melting ranges rather than a single temperature, due to their mixed composition.
Understanding this baseline is the first step in navigating the broader world of aluminum alloys and their unique thermal behaviors. Next, we’ll clarify how to express and convert the melting point of aluminum between Celsius and Fahrenheit, ensuring you can communicate these values accurately across global contexts.

When you search for the melting point of aluminum, you’ll notice it’s often listed in both Celsius and Fahrenheit. But why does this matter? Imagine you’re working on an international project, reading technical data sheets, or simply comparing oven temperatures at home—specifying the right unit makes all the difference. Let’s break down exactly what these numbers mean and how you can convert between them.
Temperature units aren’t just a matter of preference—they’re essential for clear communication. While most of the world uses Celsius, Fahrenheit remains common in the United States. Mixing them up can lead to costly mistakes or confusion, especially in engineering or manufacturing environments.
Here’s a quick reference for the melting point of aluminum in both units:
These values are rounded for simplicity, but you may also see the more precise figures: 660.32°C and 1220.58°F.
Need to switch between units? It’s easier than you might think. Here’s the formula:
°F = (°C × 9/5) + 32
°C = (°F − 32) × 5/9
For example, to convert the melting point of aluminum from Celsius to Fahrenheit:
This simple calculation ensures you can confidently interpret technical data or communicate specifications, no matter where you are in the world (reference).
Understanding how to express and convert the melting point of aluminum in celsius and fahrenheit sets the foundation for discussing how real-world products, like aluminum foil and cans, behave when exposed to heat. Up next, we’ll explore how these everyday items differ from pure aluminum when it comes to melting.
Ever wondered if your aluminum foil would melt in the oven, or how hot a soda can has to get before it turns to liquid? It’s a practical question, especially since these items are so common in kitchens and recycling bins everywhere. Let’s break down what you need to know about the melting point of aluminum foil and cans—and whether their thinness or alloy content actually changes their behavior compared to pure aluminum.
Sounds complex? Actually, the answer is straightforward: the melting point of a metal is an intrinsic property, meaning it doesn’t change whether the metal is in the form of a thick block, a thin sheet, or a delicate foil. So, the melting point of aluminum foil remains the same as that of pure aluminum—approximately 660°C (1,220°F) (source).
When it comes to the melting point of an aluminum can, things get a bit more interesting. Most beverage cans are made from aluminum alloys, not pure aluminum. These alloys are engineered for strength, corrosion resistance, and formability. Alloying elements (like magnesium or manganese) can slightly lower or broaden the melting range compared to pure aluminum, but the difference is usually not dramatic for common can alloys.
Understanding how aluminum foil and cans behave under heat helps you use them safely and recycle them effectively. But what happens when aluminum is intentionally alloyed for specialized industrial uses? Next, we’ll dive into the melting points of popular aluminum alloys—and why this matters for manufacturing and engineering.

When you move beyond pure aluminum and step into the world of alloys, things get a bit more nuanced. Ever wondered why the melting point of aluminum-6061 or the melting point of 7075 aluminum isn’t just a single number, but a range? Or how manufacturers select the right alloy for everything from aircraft frames to automotive parts? Let’s break it down in plain language and see why understanding these melting ranges is crucial for today’s industries.
Unlike pure aluminum, which melts at a precise temperature, alloys are mixtures—think of them as recipes with several ingredients. Each added element (like magnesium, silicon, copper, or zinc) changes the way the atoms bond together, resulting in a melting range rather than a single melting point. This range is defined by two key temperatures:
This is why, when you look up the melting point of aluminum-6061 or the melting point of 7075 aluminum, you’ll see a span of temperatures rather than one fixed value.
To make things clearer, here’s a comparison table of several widely used aluminum alloys, their principal alloying elements, and their typical melting ranges in both Celsius and Fahrenheit:
| Alloy Designation | Key Alloying Elements | Melting Range (°C) | Melting Range (°F) |
|---|---|---|---|
| 6061 | Magnesium, Silicon | 582 – 651 | 1080 – 1204 |
| 7075 | Zinc, Magnesium, Copper | 477 – 635 | 891 – 1175 |
| 3003 | Manganese | 644 – 654 | 1191 – 1219 |
| 5052 | Magnesium, Chromium | 607 – 649 | 1125 – 1200 |
| 6063 | Magnesium, Silicon | 600 – 655 | 1112 – 1211 |
Imagine you’re designing a machine part that must withstand high temperatures, or you’re selecting a material for a structural beam in a new building. The specific melting range of your chosen alloy determines:
For example, the melting point of aluminum-6061 (582–651°C) makes it ideal for structural applications, while the lower melting range of 7075 (477–635°C) is balanced by its exceptional strength, making it a go-to for aerospace and high-performance engineering.
With so many variables at play, selecting the right aluminum alloy profile is a job for experts. That’s why leading manufacturers and innovators turn to trusted partners like Shengxin Aluminum. Their deep experience in producing custom alloy profiles—backed by advanced extrusion lines and rigorous quality controls—ensures every project gets the optimal blend of strength, formability, and thermal performance required for success in sectors like transportation, construction, and electronics.
Understanding the melting ranges of common aluminum alloys doesn’t just help you pick the right material—it empowers smarter design, safer manufacturing, and better end products. Next, we’ll explore the factors that cause these melting points to vary, giving you even more insight into the science behind alloy selection and performance.
When you look at the melting point of aluminum alloys, you’ll notice it’s rarely a single, neat number. Why do these alloys melt over a range of temperatures, and what makes one alloy different from another? Let’s break down the science behind these variations, so you can make sense of the numbers you see on datasheets or in engineering specs.
Imagine baking a cake—change the ingredients, and you get a different flavor and texture. The same goes for aluminum alloys. Here’s what influences the melting point of an aluminum alloy:
Understanding why the melting point of aluminum alloys varies is more than academic—it’s practical. Selecting the right alloy for casting, welding, or forming depends on knowing its melting behavior. For instance, if you need an alloy that flows easily into a mold, you might look for one with a lower melting range and good fluidity (often achieved with higher silicon content). On the other hand, structural applications exposed to heat may require alloys with higher melting ranges and fewer impurities.
Now that you know what drives the melting point of aluminum alloy up or down, you’re better prepared to select the right material for your next project. Up next, we’ll see how aluminum’s melting point stacks up against other everyday metals like steel and copper—and why those differences matter in real-world applications.
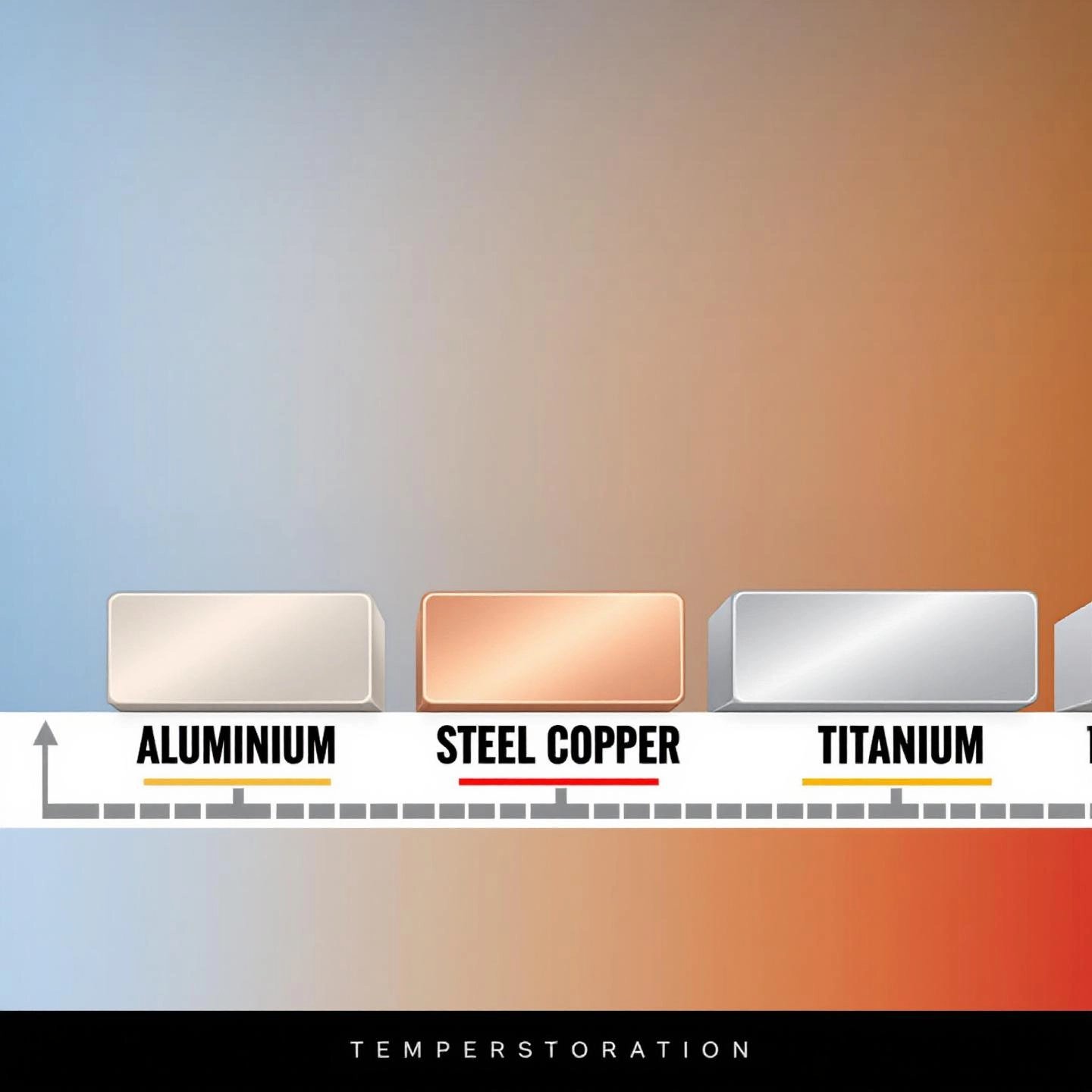
When you’re choosing the right metal for a project—whether it’s building a bridge, wiring a house, or designing a jet engine—one of the first questions you’ll face is, “How does this metal handle heat?” The melting point of aluminum vs steel or copper isn’t just a number; it’s a deciding factor in everything from manufacturing methods to safety standards. Let’s break down how aluminum stacks up against some of the most common metals you encounter in industry and everyday life.
Imagine you’re comparing building blocks for a high-temperature environment. Would you pick aluminum, with its low melting point, or opt for something like steel or titanium? Here’s a side-by-side look at the melting points of key metals, with values provided in both Celsius and Fahrenheit for clarity (Fractory):
| Metal | Melting Point (°C) | Melting Point (°F) | Notes |
|---|---|---|---|
| Aluminum (pure) | 660 | 1220 | Lightweight, easy to melt and cast |
| Copper | 1084 | 1983 | Excellent electrical and thermal conductivity |
| Carbon Steel | 1371–1593 | 2500–2900 | High strength, melting range depends on carbon content |
| Stainless Steel | 1510 | 2750 | Corrosion resistant, higher melting than carbon steel |
| Titanium | 1670 | 3040 | Exceptional strength-to-weight ratio, high melting point |
Still wondering why the melting point of aluminum vs steel is so important? Here’s how these numbers play out in real life:
So, when you’re deciding between aluminum, steel, or copper, consider not just the numbers, but what they mean for your application. Aluminum’s lower melting point makes it easy to work with and recycle, but limits its use in high-heat settings. Steel and titanium open the door to demanding, heat-intensive applications, while copper finds its niche in electrical and thermal systems.
Next, let’s look at how aluminum’s melting point compares to its compounds—like aluminum oxide—and why these differences are crucial for advanced applications.
When you think about the melting point of aluminum, you might picture a silvery metal liquefying at 660°C (1,220°F). But what happens when aluminum is combined with other elements to form compounds? The answer: the melting points can change dramatically—sometimes reaching temperatures far beyond what pure aluminum can withstand.
Imagine you’re designing a furnace lining or a protective coating for a jet engine. Would pure aluminum work? Not quite—because it would melt at relatively low temperatures. Instead, you’d turn to aluminum oxide (also called alumina, Al2O3), a compound with a melting point that soars to about 2,072°C (3,762°F) (Wikipedia: Aluminium oxide).
So, while pure aluminum would fail at high temperatures, aluminum oxide stands strong—making it a go-to material for demanding environments.
Not all aluminum compounds are created equal when it comes to melting points. Take aluminum chloride (AlCl3) as an example—it melts at a much lower temperature, around 192.6°C (378.7°F), and is used primarily as a catalyst in chemical manufacturing. Its low melting point reflects the weaker bonds between aluminum and chlorine compared to the strong aluminum-oxygen bonds in alumina.
Understanding the melting point of aluminum oxide and other compounds helps engineers and scientists pick the right material for every challenge—whether it’s withstanding extreme heat, resisting corrosion, or catalyzing complex reactions. Next, we’ll explore how these melting points influence practical manufacturing processes like casting and brazing, and why selecting the right alloy or compound is crucial for success.
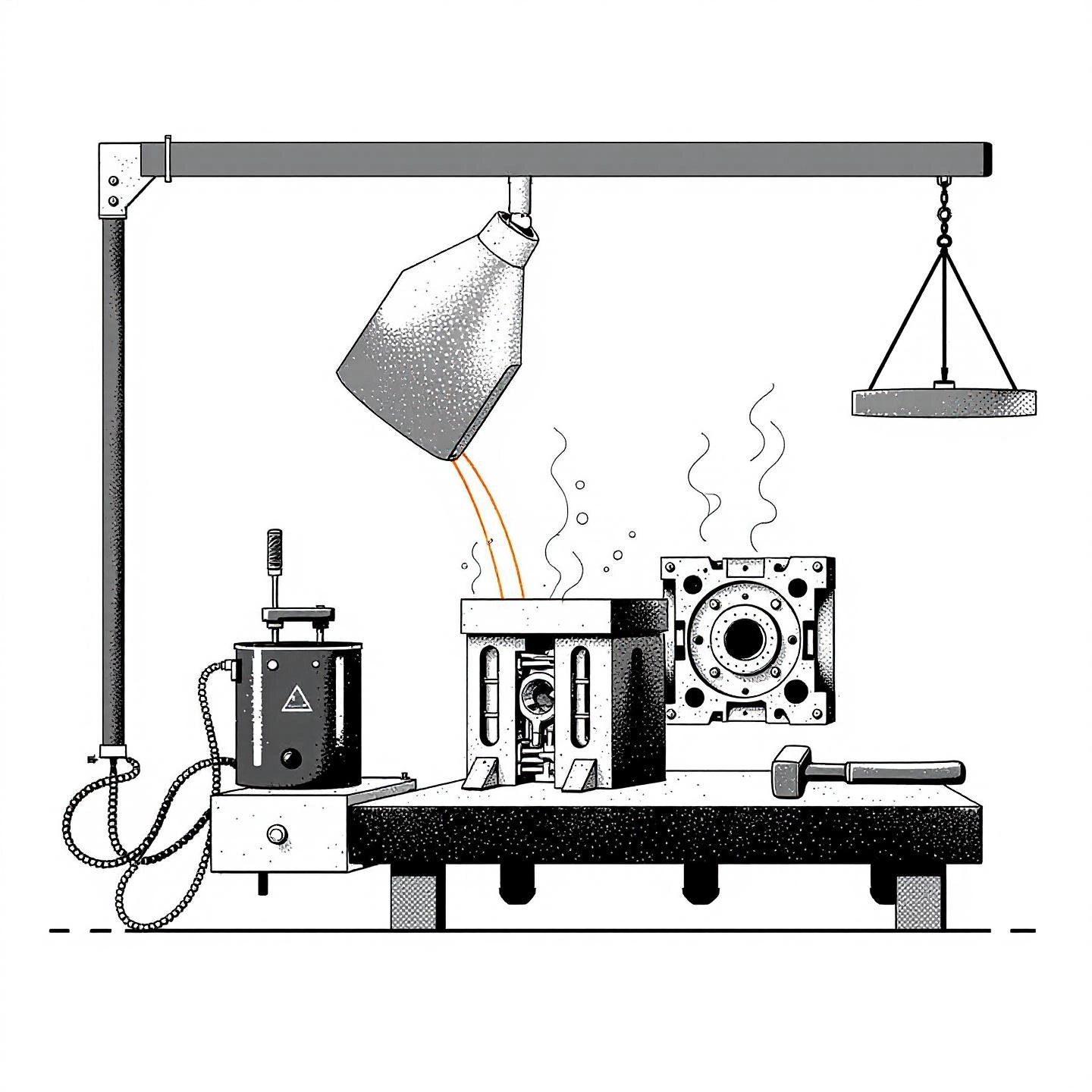
When you picture molten aluminum flowing into a mold or a brazing rod joining two metal pieces, it’s easy to overlook the science behind the scenes. Yet, understanding the melting point of cast aluminum and the melting point of aluminum brazing rod is essential for manufacturers, engineers, and even hobbyists aiming for strong, defect-free results. Why? Because the right temperature isn’t just a number—it’s the foundation for quality, safety, and efficiency in every aluminum project.
Imagine pouring liquid metal into a mold. If the temperature is too low, the metal may solidify before filling every corner, leading to shrinkage defects or incomplete parts. Too high, and you risk hot cracks, gas porosity, or even damaging your mold. That’s why knowing the precise melting point—and the range for your specific alloy—is critical.
When it comes to joining aluminum parts, brazing is a popular technique. The key is to use a brazing rod that melts at a lower temperature than your base metals—otherwise, you risk melting or warping the parts you’re trying to join.
Precision and consistency in thermal management are not just technical details—they’re the difference between a flawless component and costly rework. For projects that demand custom aluminum profiles, tight tolerances, and advanced processing, partnering with a manufacturer equipped for high standards is essential. Shengxin Aluminum stands out with over 100 production lines, including cutting-edge extrusion, deep processing, and CNC machining capabilities. This scale and expertise ensure that every profile meets exact thermal and mechanical requirements, whether you’re casting intricate parts or brazing high-performance assemblies(Shengxin Aluminum).
Understanding the melting point of cast aluminum and the role of brazing rods empowers you to make smarter choices in design, manufacturing, and repair. Next, we’ll wrap up with a summary of key takeaways—and how expert partners can help you achieve the best results for your aluminum projects.
When you think about the role aluminum plays in your world—whether it’s the foil in your kitchen or the high-speed trains and skyscrapers shaping our cities—its melting point is at the heart of that versatility. Let’s quickly recap the essential insights you’ve gained about the melting point of aluminum and why this property matters so much for both everyday users and forward-thinking industries.
Imagine designing a building that’s both strong and lightweight, or a vehicle that’s fuel-efficient yet durable. Aluminum’s unique combination of a moderate melting point, corrosion resistance, and adaptability to alloying makes it the go-to material for these challenges. Its ability to be extruded, formed, and recycled again and again means it’s not just a metal of today—it’s a building block for the future.
Whether you’re specifying aluminium profiles for architectural projects or sourcing industrial aluminium profiles for transportation, energy, or electronics, the right expertise makes all the difference. A knowledgeable partner ensures your profiles are engineered with precise melting ranges, mechanical strength, and performance tailored to your needs.
That’s where leading manufacturers like Shengxin Aluminum stand out. With extensive production capabilities, advanced extrusion lines, and a reputation for reliability, they deliver high-quality, custom aluminum solutions trusted by innovators in demanding sectors. Their commitment to quality, technical support, and continuous innovation ensures your projects benefit from the full potential of aluminum—no matter how complex the requirements.
In summary, understanding the melting point of aluminum isn’t just about numbers—it’s about unlocking new possibilities, ensuring safety, and driving sustainable progress. Whether you’re a designer, engineer, or simply curious about the materials that shape our world, you’re now equipped to make smarter choices and achieve better results with aluminum at your side.
Aluminum foil melts at around 660°C (1,220°F), the same as pure aluminum. Household ovens and grills do not reach these temperatures, so foil will not melt during typical cooking or baking. The thinness of foil means it heats quickly, but its melting point remains unchanged regardless of thickness.
Tungsten is known for its extremely high melting point of 3,422°C (6,192°F), making it one of the most heat-resistant metals. In comparison, aluminum melts at much lower temperatures, which is why tungsten is used for applications needing exceptional heat resistance, such as filaments and high-temperature tools.
Yes, aluminum alloys and products like cans have melting ranges rather than a single melting point. Alloying elements such as magnesium, zinc, or copper lower or broaden the melting range, but most aluminum cans still melt close to the pure aluminum point. The exact temperature depends on the specific alloy composition.
Knowing the melting point is crucial for processes like casting, brazing, and extrusion. It ensures optimal temperatures are used for forming, joining, and shaping aluminum, which helps prevent defects and maximizes product quality. Manufacturers like Shengxin Aluminum use this knowledge to produce high-quality, custom profiles for demanding industries.
Aluminum melts at 660°C (1,220°F), which is significantly lower than copper (1,084°C/1,983°F) and steel (1,371–1,593°C/2,500–2,900°F). This lower melting point makes aluminum easier to process and recycle but limits its use in high-temperature applications compared to steel or copper.
 serviço on-line
serviço on-line 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360