
Have you ever wondered, “Is aluminum a metal?” If so, you’re not alone. This question pops up regularly for students tackling science homework, DIYers choosing materials for their next project, and professionals in fields from engineering to construction. The answer is straightforward: yes, aluminum is a metal. But why is this question even up for debate?
Aluminum’s unique characteristics—like its silvery shine, light weight, and resistance to rust—sometimes make people second-guess whether it fits the typical image of a metal. Unlike iron or copper, it’s rarely found in its pure form in nature, and its everyday uses range from soda cans to airplane parts. These factors can make aluminum seem a bit mysterious, leading to the common query: is aluminum a metal, yes or no? The answer, as you’ll see, is a resounding yes, confirmed by both science and industry experts.
This article will guide you through the science behind aluminum’s metallic status. We’ll break down what defines a metal, explore aluminum’s physical and chemical properties, and compare it to other types of elements—like metalloids and nonmetals. You’ll also learn about its place on the periodic table, its magnetic and ferrous qualities, density, malleability, and practical ways to identify it in your daily life.
Let’s dive in and uncover the simple scientific answer to whether aluminum is a metal—and why it matters for so many of us.
Get answers to all these questions – and a deeper understanding of aluminum’s true nature – in our comprehensive guide: Is Aluminum a Metal? Your Definitive Guide to Its True Nature.
This detailed resource explores the science behind what defines a metal, delves into aluminum’s key properties (like its place on the periodic table, density, malleability, and magnetic behavior), and provides clear ways to identify aluminum in everyday objects. Whether you're a student, DIYer, or professional, this guide gives you the clarity you need about this essential material.
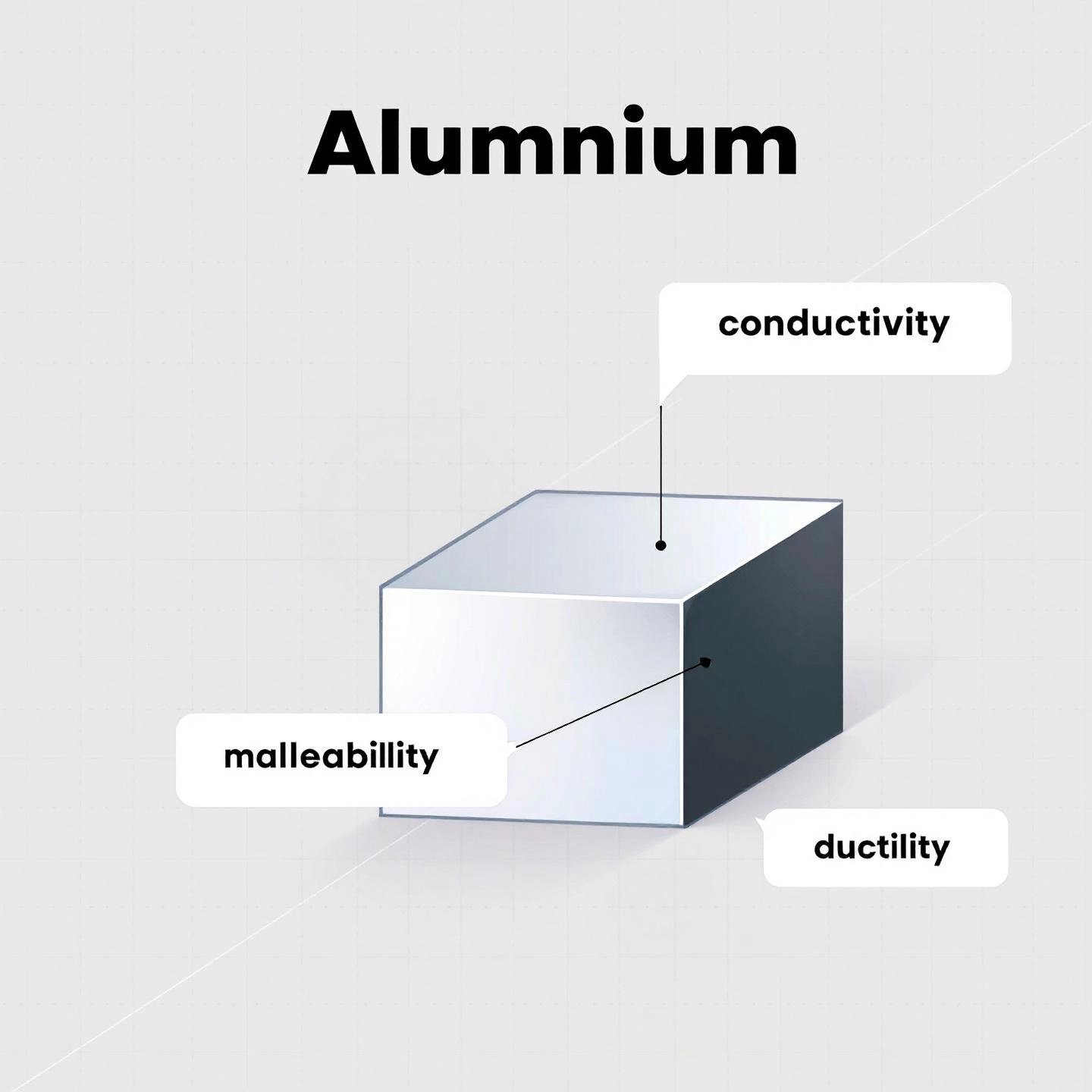
When you picture a metal, what comes to mind? Maybe it’s the shine of a steel beam, the feel of a copper wire, or the weight of an iron skillet. But how do scientists actually define what makes something a metal? Let’s break down the key characteristics that set metals apart—and see exactly how aluminum fits right in.
In chemistry and physics, metals are defined by a specific set of physical and chemical properties. Here’s a quick checklist to help you spot a metal, whether you’re in the lab or at home:
Now, let’s see how aluminum stacks up against each of these classic metal properties:
Given all these properties, it’s easy to see why aluminum is classified as a metal both in scientific terms and in everyday use. If you check the periodic table, you’ll find aluminum in Group 13, clearly labeled as a metal. Its atomic number is 13, and it shares its group with other metals like gallium and indium.
So, next time you wonder about aluminum properties or ask, “is aluminum a metal on the periodic table?”—you’ll know that it checks every box for what makes a substance a true metal. This solid foundation helps us compare aluminum to other types of elements, like metalloids and nonmetals, which we’ll explore in the next section.
When you examine the periodic table, you’ll notice that not all elements are created equal. Some shine and conduct electricity, while others are dull and insulate. This leads to a common question: is aluminum a metal or metalloid? Or perhaps, is aluminum a metal or nonmetal? The answer lies in understanding the fundamental differences between these three major groups of elements.
Let’s break down what each group means in simple terms:
To make things clearer, here’s a table comparing the key properties of metals, metalloids, and nonmetals—and showing exactly where aluminum belongs:
| Property | Metals (e.g., Aluminum) | Metalloids | Nonmetals |
|---|---|---|---|
| Appearance | Shiny, silvery, or metallic luster | Can be shiny or dull | Dull, non-lustrous |
| Electrical Conductivity | Excellent (Aluminum is widely used in power lines) | Moderate to poor | Poor |
| Thermal Conductivity | High (Aluminum pans heat quickly and evenly) | Moderate | Poor |
| Malleability | High (Aluminum can be formed into foil or cans) | Brittle or semi-malleable | Brittle if solid |
| State at Room Temperature | Solid (except mercury) | Solid | Solid, liquid, or gas |
| Position on Periodic Table | Left and center (Aluminum: Group 13) | Stair-step line between metals and nonmetals | Right side (except hydrogen) |
| Examples | Aluminum, copper, iron | Silicon, boron, arsenic | Oxygen, nitrogen, sulfur |
So, is aluminum a metal or metalloid? As the table shows, aluminum matches all the classic metal traits: it’s shiny, lightweight, highly malleable, and an excellent conductor of heat and electricity (ChemistryTalk). It sits firmly in Group 13 of the periodic table, surrounded by other metals. Unlike metalloids, aluminum is not brittle and does not have only moderate conductivity. And compared to nonmetals, it’s the opposite in almost every property—nonmetals are dull, poor conductors, and often found as gases or brittle solids.
In summary, aluminum is not a metalloid or nonmetal. It’s a true metal by both scientific definition and everyday experience. Understanding these distinctions makes it easier to see why aluminum is so widely used in everything from construction to cookware. Next, let’s pinpoint exactly where aluminum sits on the periodic table and what that means for its classification.
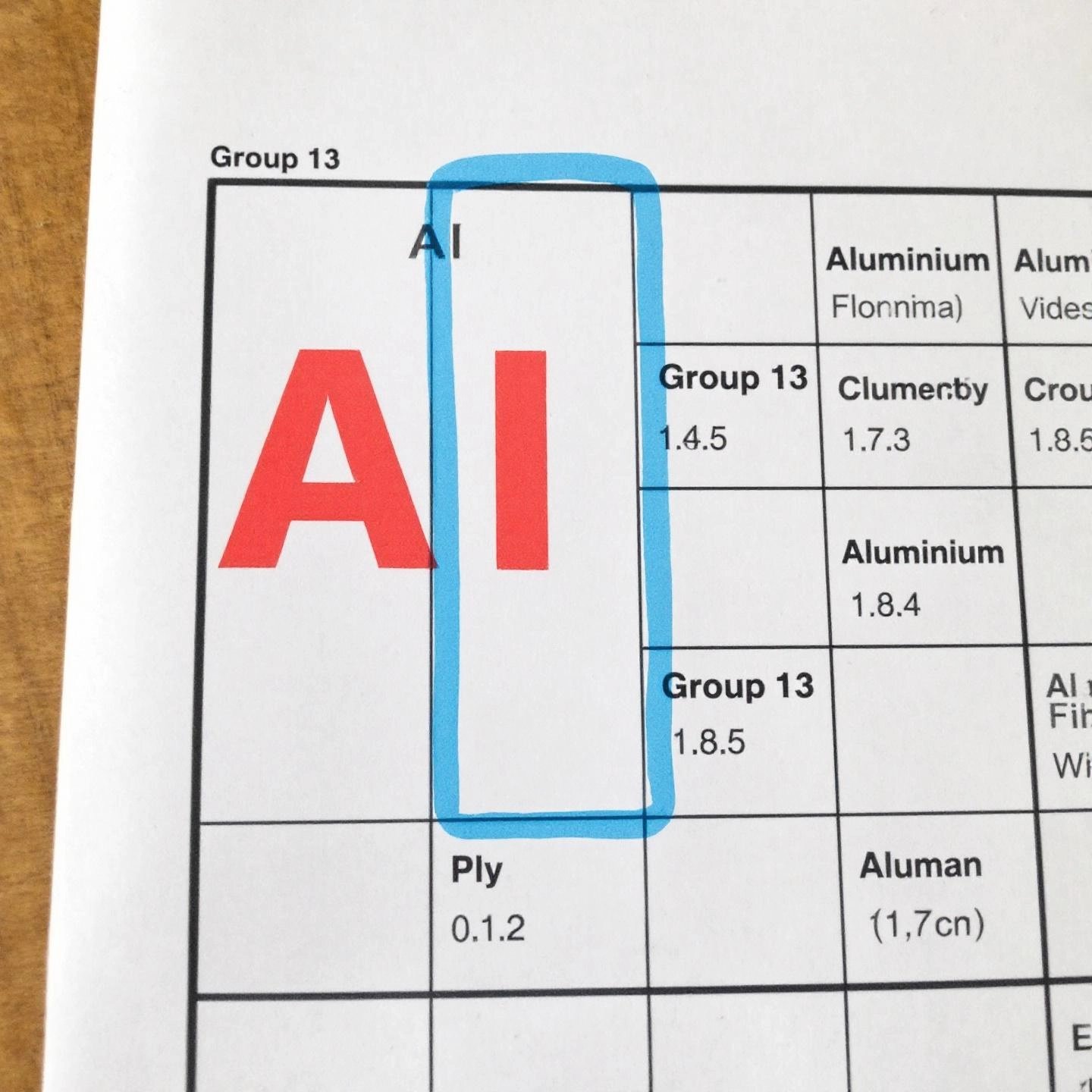
Ever wondered exactly where aluminum fits on the periodic table? If you’re searching for answers to “is aluminum a transition metal” or want to understand its scientific classification, you’re in the right place. Let’s break it down in a way that’s easy to remember—and see why aluminum stands out among the elements.
When you look at the aluminum periodic table entry, you’ll find it in the third row from the top (Period 3) and in the thirteenth column from the left (Group 13). This group also includes elements like boron, gallium, indium, and thallium (ThoughtCo).
This is a common question, especially since aluminum is so widely used in industry. The answer is: no, aluminum is not a transition metal. Here’s why:
Instead, aluminum is classified as a post-transition metal. This means it comes after the transition metals on the periodic table and shares some—but not all—metallic traits. It’s lighter, less dense, and lacks the complex chemistry of true transition metals.
Aluminum, on the other hand, most commonly forms compounds in a +3 oxidation state and is generally colorless in its ionic form. So, if you’re ever asked “is aluminum a transition metal?” you can confidently say it’s not—its place is as a post-transition metal in Group 13.
Now that you know exactly where aluminum sits on the periodic table and how it’s classified, let’s explore another fascinating property: its magnetic behavior—and why that matters for everyday use.
When you pick up a refrigerator magnet and press it against an aluminum can, you’ll notice—nothing happens. So, is aluminum magnetic? The answer isn’t as black and white as you might think, but it’s surprisingly simple once you understand the science behind it.
Let’s start with the basics. Most people are familiar with ferromagnetism—the strong, permanent magnetism you see in metals like iron and steel. These materials are powerfully attracted to magnets and can even become magnets themselves. But not all metals behave this way.
So, where does aluminum fit? Aluminum is classified as paramagnetic. This means it has unpaired electrons that align ever-so-slightly with a magnetic field, but the effect is so weak that you won’t notice it in daily life.
Imagine holding a powerful magnet next to a piece of aluminum. Technically, there’s a tiny attraction—so small, in fact, that it’s almost undetectable unless you use specialized equipment or extremely strong magnets. As soon as you remove the magnet, aluminum immediately loses any trace of magnetism. For all practical purposes, aluminum is non-magnetic.
Here’s a fun experiment: Drop a strong magnet down an aluminum tube, and you’ll see the magnet fall slowly. This isn’t because aluminum is magnetic, but because of something called Lenz’s Law. The moving magnet induces a current in the aluminum, creating a temporary, opposing magnetic field that slows the fall. Still, the aluminum itself isn’t truly magnetic—it just reacts briefly to the changing field.
So, to answer the big question: Is aluminum magnetic? No, not in any way you’d notice in everyday life. It’s a metal, but its paramagnetic property means it remains, for all practical purposes, non-magnetic. This sets aluminum apart from metals like iron and helps explain why it’s so valuable in industries where magnetic interference must be avoided. Next, let’s look at another essential distinction: whether aluminum is considered a ferrous or non-ferrous metal—and what that means for its uses.
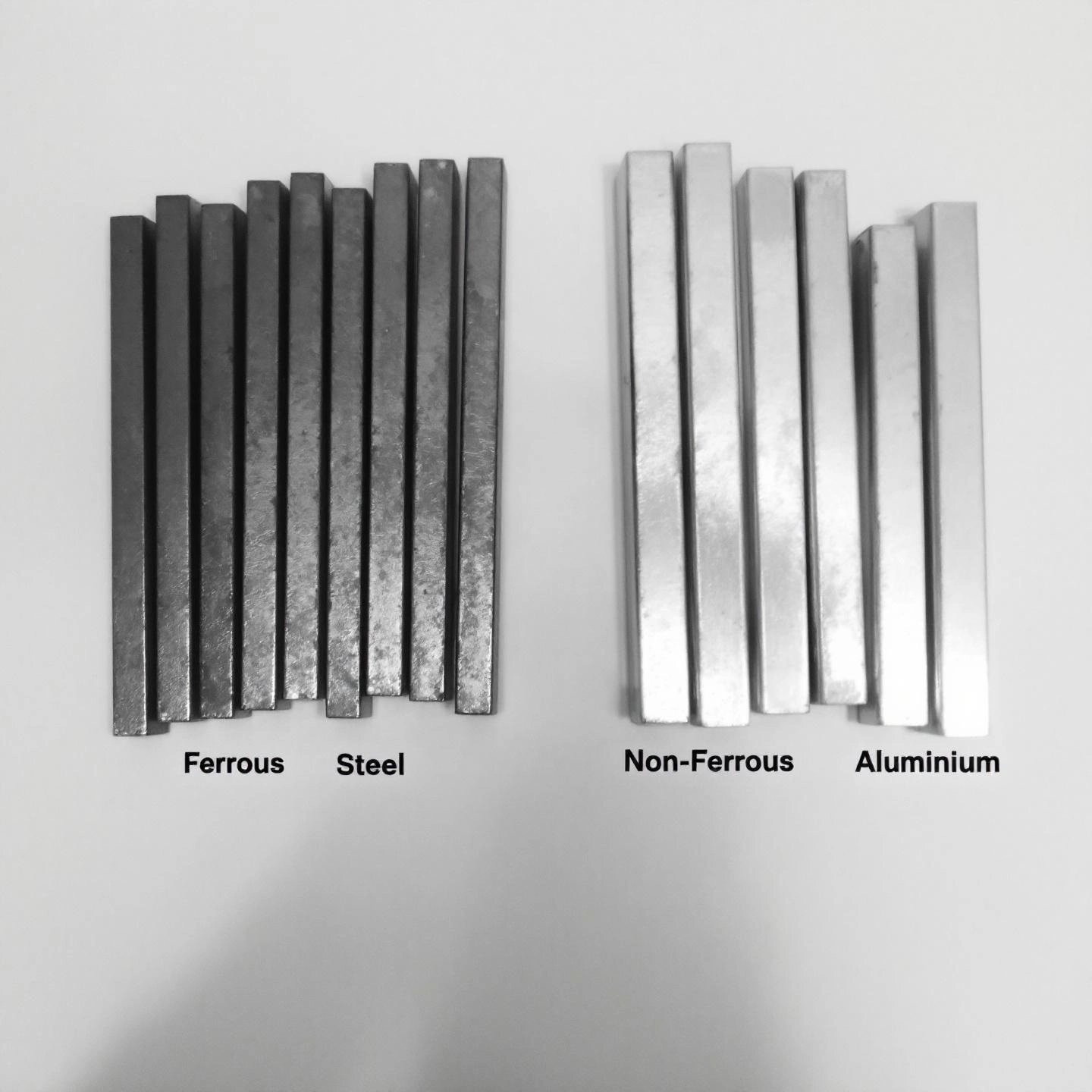
Ever wondered, is aluminum a ferrous metal? The answer is clear: aluminum is not a ferrous metal—it’s a classic example of a non-ferrous metal. But what does that actually mean, and why does it matter for your next project or purchase?
Let’s start with the basics. Ferrous metals are those that contain iron as a main ingredient. Think of steel beams, iron skillets, or the frame of a car—these materials are strong, often magnetic, and prone to rust if left unprotected. In contrast, non-ferrous metals contain little to no iron. This single distinction leads to a whole new set of properties and advantages.
So, aluminum is a non-ferrous metal because it has virtually no iron in its composition. This sets it apart from common building metals like steel and gives it a unique edge in many industries.
Why choose a non-ferrous metal like aluminum over traditional ferrous options? Let’s break down the main advantages that make aluminum a favorite for engineers, builders, and manufacturers worldwide:
Imagine you’re designing a modern, energy-efficient building. You need window frames that won’t rust, are easy to install, and help keep energy costs down. Or maybe you’re tasked with building lightweight, corrosion-resistant components for the next generation of electric vehicles or solar panels. In all these scenarios, aluminum’s non-ferrous nature gives it a clear advantage.
Leading manufacturers like Shengxin Aluminum harness these properties to produce high-quality aluminum profiles for everything from eco-friendly curtain walls and energy-saving windows to advanced industrial components. Because aluminum won’t rust, stays strong yet light, and can be shaped into almost any form, it’s the go-to choice for applications where performance and longevity matter.
So, the next time you’re comparing materials for a project, remember the unique benefits of non-ferrous metals—especially aluminum. Its combination of lightness, corrosion resistance, and versatility makes it indispensable across countless industries. Up next, let’s explore how aluminum’s density compares to so-called "heavy metals," and why it’s considered a lightweight champion in the metal world.
When you hear the term “heavy metal,” what comes to mind? Maybe it’s the image of dense, toxic substances or the booming sound of a rock band. But in science, “heavy metal” has a specific meaning tied to density and atomic weight. So, is aluminum a heavy metal? Let’s break it down in simple terms so you can confidently answer this question.
In scientific circles, heavy metals are typically defined as elements with high densities (usually above 5 g/cm³), high atomic numbers, or high atomic masses. These metals—like lead, mercury, and gold—are physically heavy for their size and often have significant environmental or health concerns associated with them.
Aluminum stands out for its low density—just about 2.7 g/cm³ (RIT Density Table). That’s less than half the density of iron, and far below traditional heavy metals. To make this clearer, here’s how aluminum stacks up against some common metals:
| Metal | Density (g/cm³) | Classification |
|---|---|---|
| Aluminum | 2.7 | Light metal |
| Iron | 7.85 | Base/heavy metal |
| Lead | 11.3 | Heavy metal |
| Mercury | 13.6 | Heavy metal |
| Gold | 19.3 | Heavy metal |
As you can see, aluminum’s density is much lower than that of iron, lead, or mercury. This is why it’s classified as a light metal, not a heavy metal.
So, if you’re comparing materials for a project and wondering about aluminum density, keep in mind: aluminum is prized for being lightweight, easy to handle, and strong for its size. It’s not considered a heavy metal by any scientific standard.
This lightweight nature is a major reason aluminum is used in everything from airplanes to window frames—where strength and low weight are essential. Up next, let’s see how aluminum’s softness and malleability make it even more versatile in real-world applications.
When you think of metals, you might picture something hard and unyielding—like the steel beams supporting a skyscraper. But what happens when a metal is soft and exceptionally easy to shape? That’s exactly where aluminum stands out. Its remarkable malleability is one of the reasons it’s so widely used across industries, from your kitchen to high-speed trains.
Aluminum’s atomic structure allows its atoms to slide past one another with minimal resistance. This means it can be pressed, rolled, or hammered into thin sheets without cracking—imagine how easily aluminum foil bends and wraps around your leftovers. In fact, compared to harder metals like steel, aluminum is far less likely to fracture when formed into complex shapes.
One of the most important ways manufacturers harness aluminum’s malleability is through aluminum extrusion. This process pushes heated aluminum through a shaped die, creating long, continuous pieces with precise cross-sections—think of it like squeezing toothpaste from a tube, but with metal. The result? Custom profiles and components that are both lightweight and strong, tailored for everything from window frames to automotive parts.
Benefits of aluminum extrusion include:
You’ll find aluminum’s softness and malleability at work all around you. At home, it’s in beverage cans, kitchen foil, and even furniture. In industry, aluminum extrusion is essential for building construction, transportation, and electronics. For example, the frames of modern trains and buses, intricate heat sinks in computers, and even architectural features like curtain walls all rely on aluminum’s unique ability to be shaped and formed without losing strength.
Advanced manufacturers, such as Shengxin Aluminum, leverage this property to deliver high-quality, custom aluminum profiles for demanding sectors like transport and construction. By utilizing state-of-the-art extrusion technology, they’re able to produce complex, durable components that meet the precise needs of modern engineering projects.
So, next time you unwrap a sandwich or ride a subway, remember: it’s aluminum’s softness and malleability that make these innovations possible. In the next section, we’ll look at practical ways you can identify aluminum in your daily life—whether you’re at home, in the workshop, or on the job site.

Ever wondered if that shiny metal part in your hand is aluminum or something else? Whether you’re a DIY enthusiast, a student, or just sorting through recyclables, knowing how to identify aluminum can save time and prevent costly mistakes. With a few easy tests and some everyday know-how, you’ll be able to spot aluminum at home, in the workshop, or on the job site.
Sounds complex? Actually, identifying aluminum is straightforward when you know what to look for. Here are the most practical methods you can use—no special tools required:
Still not sure? Here are some classic examples where you’ll encounter aluminum versus steel or other metals:
Imagine you’re sorting scrap metal or picking materials for your next home improvement project. A quick magnet test, a scratch with your key, or just noticing the weight can tell you a lot about what you’re holding. And if you’re ever in doubt—especially with large or valuable pieces—professional metal services can use advanced tools like X-ray fluorescence (XRF) for precise identification.
Knowing these simple tricks not only helps you tell aluminum vs steel apart, but also empowers you to choose the right material for the job. Next, we’ll wrap up by summarizing aluminum’s key features and why it’s so essential in today’s world.
After exploring its scientific definition, properties, and real-world applications, the answer to the question is aluminum a metal is crystal clear: yes, aluminum is unequivocally a metal. From its position in Group 13 of the periodic table to its unmistakable combination of luster, conductivity, and malleability, aluminum checks every box that defines what a metal is (Britannica).
These unique features explain why aluminum uses are so widespread—from transportation and construction to packaging, consumer goods, and advanced technology. Whether you’re designing energy-efficient buildings, manufacturing lightweight vehicles, or simply wrapping leftovers, aluminum’s versatility and reliability make it a material of choice worldwide.
Imagine the possibilities when you select the right aluminum profile or component—lighter vehicles, longer-lasting windows, and energy savings that add up over time. For those seeking top-tier quality and precision, manufacturers like Shengxin Aluminum offer a broad range of expertly engineered aluminum products for everything from eco-friendly construction to high-tech industrial solutions.
So, the next time you ask yourself, "is aluminum a metal?"—remember the science, the properties, and the everyday innovations it makes possible. If you’re planning your next project or business venture, consider the lasting value and performance of high-quality aluminum. It’s more than just a metal—it’s a foundation for progress in the modern world.
Yes, aluminum is classified as a metal. It is recognized for its luster, high electrical and thermal conductivity, and malleability. On the periodic table, aluminum is found in Group 13 and is widely used in industries due to its lightweight and corrosion-resistant properties.
Aluminum is an elemental metal, meaning it consists of only aluminum atoms and is not a mixture. While it can be alloyed with other metals for specific applications, pure aluminum itself is entirely a metal.
Aluminum is a metal, not a metalloid. It exhibits all the core characteristics of metals, such as being shiny, conductive, malleable, and solid at room temperature. Unlike metalloids, aluminum is not brittle and is an excellent conductor.
Aluminum is non-ferrous because it contains little to no iron. This gives it advantages like resistance to rust, a lighter weight, and non-magnetic properties, making it ideal for applications such as energy-efficient windows, transportation, and advanced industrial profiles.
You can recognize aluminum by its light weight, non-magnetic nature, and silvery appearance. It does not rust like iron; instead, it forms a white oxide layer. Common items made from aluminum include cans, foil, window frames, and some bike parts.
 serviço on-line
serviço on-line 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360